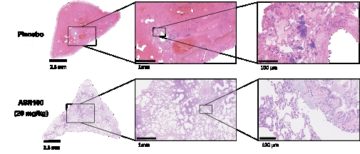
Antimicrobial / antibiofilm efficacy
- Antibiogram
- Minimum Inhibitory Concentration (MIC) – liquid or solid method
- Minimum Bactericidal Concentration (MBC)
- Time Kill Curve (TKC)
- Fractionated Inhibitory Concentration (FIC) : synergic, adverse or additive effects
- Mutant Prevention Concentration (MPC)
- Ex vivo bacterial quantification, mutants investigation
- Bacterial identification (MALDI-TOF)
- Biofilm quantification / assessment of antibiofilm properties
- Titration of phages suspensions