Presentation
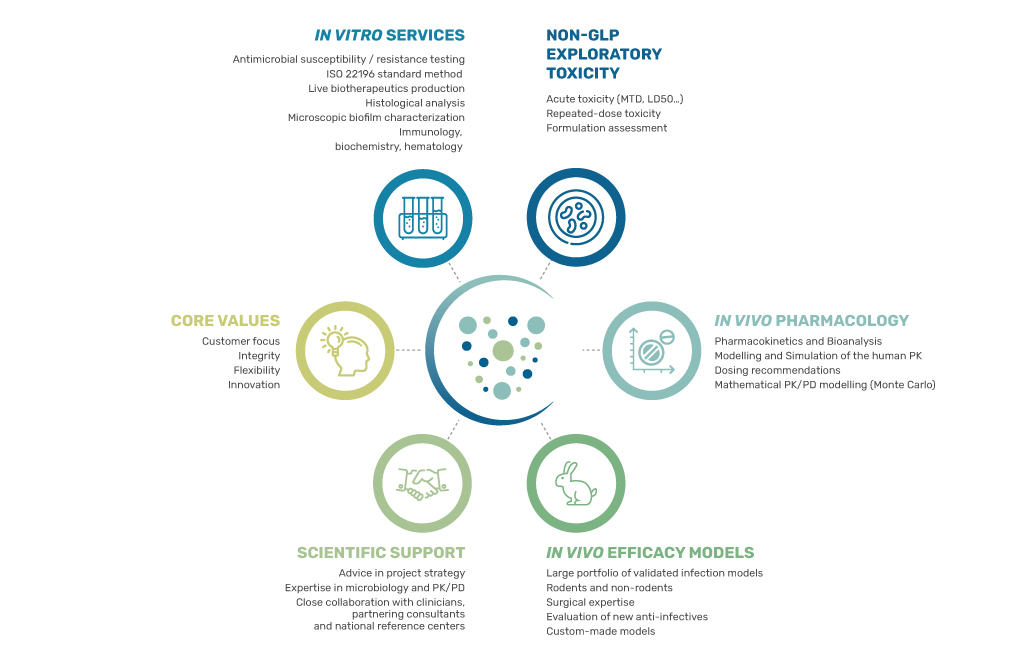
Established in 2009, VIVEXIA is a CRO (Contract Research Organization) focusing on life sciences and health. Our actions and motivation are focused on combatting antimicrobial resistant bacteria. To this aim, we specialized in the creation of humanized or non-humanized preclinical infection models that are designed for the evaluation of anti-infective therapies.
The development of our humanized models allows us to provide reliable and objective preclinical evaluation of the efficacy of treatments. This approach contributes to the selection of the very best drug candidates being clinically evaluated, notably for patients with no further therapeutic options. Our commitment to public benefit is our main motivating force and is bolstered by our close collaboration with the department for Infectious Diseases at the University Hospital in Dijon-Burgundy.
See our in vivo models See our in vitro models